
SIPM Laboratory Information Management System
In today's highly digital era, laboratory management faces challenges such as massive data volumes, complex processes, and stringent compliance requirements. LIMS (Laboratory Information Management System) is an information platform specifically designed for laboratory data management, process control, and resource optimization. By leveraging digital technologies, it integrates key aspects such as sample management, experimental workflows, data analysis, and report generation. This enables enterprises to achieve automated data handling, standardized processes, and optimized resource utilization, thereby improving efficiency, reducing costs, and ensuring compliance.
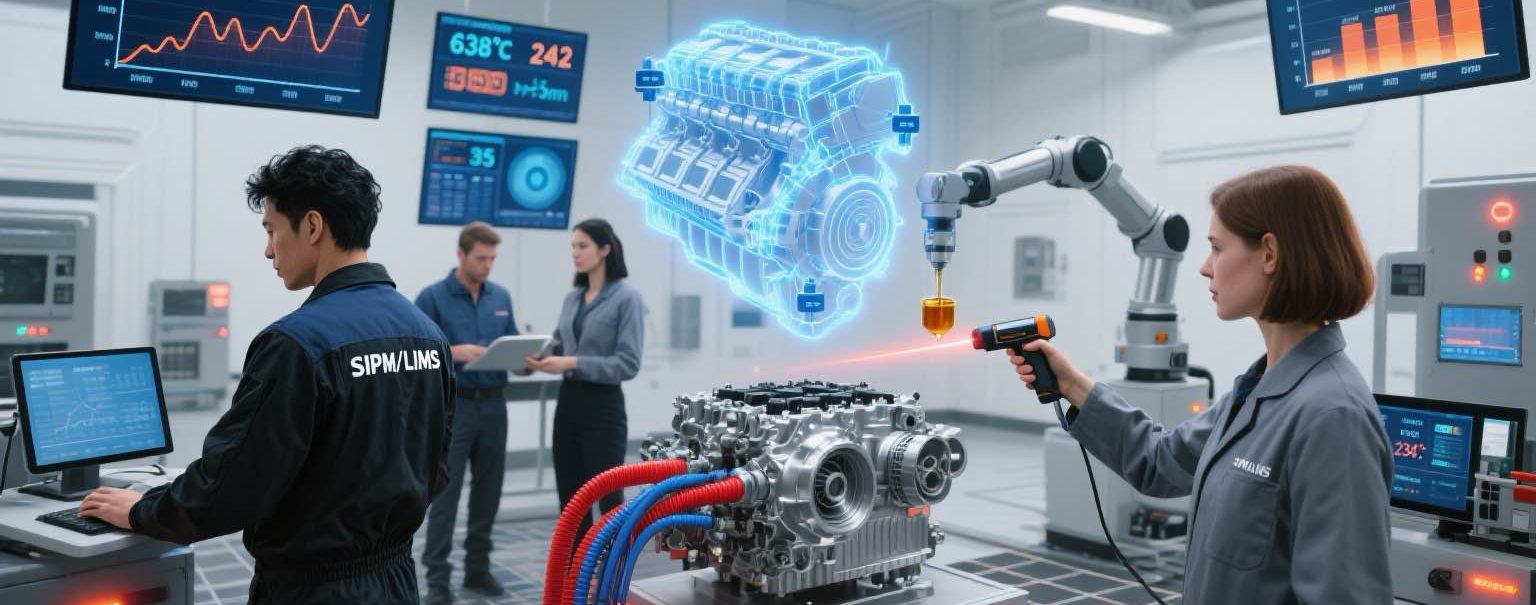










- Supports centralized management of multiple laboratories within a group
- Supports customized experimental data formats
- Supports customized experiment report templates
- Supports digital sample management
- Supports AI-powered data recognition
- Supports customized dashboard displays
- Supports integration with DingTalk and WeChat Work







Aligned with the characteristics of industry inspection and testing, integrates management requirements such as CMA and CNAS, and comprehensively meets the management elements of ISO/IEC 17025 for general laboratory competence.


Sample and prototype testing is a crucial part of the new product development process. Integrating it into the product lifecycle management (PLM) system solution enables a closed-loop implementation of the R&D V-model. Users can directly initiate sample inspection requests and access test progress and inspection data within SIPM/PLM.
_1757381527013.png)
Usher in a New Era of Intelligent Laboratory Management!
Choosing SIPM/LIMS is not just selecting a system, but embracing a future of efficient, compliant, and intelligent laboratory management!
- Online Communication
